Samarium(III) chloride

| |
| |
| Names | |
|---|---|
| IUPAC name
samarium(III) chloride
| |
| Other names
samarium trichloride
trichlorosamarium | |
| Identifiers | |
| |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.030.712 |
| EC Number |
|
PubChem CID
|
|
| UNII |
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| SmCl3 | |
| Molar mass | 256.76 g/mol (anhydrous) 364.80 g/mol (hexahydrate) |
| Appearance | pale yellow solid (anhydrous)
cream-coloured solid (hexahydrate) |
| Density | 4.46 g/cm3 (anhydrous)
2.383 g/cm3 (hexahydrate) |
| Melting point | 682 °C (1,260 °F; 955 K) |
| Boiling point | decomposes |
| 92.4 g/100 mL (10 °C) | |
| Structure | |
| hexagonal, hP8 | |
| P63/m, No. 176 | |
| Tricapped trigonal prismatic (nine-coordinate) | |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards
|
Irritant |
| GHS labelling: | |

| |
| Warning | |
| H315, H319 | |
| P264, P280, P302+P352, P305+P351+P338, P321, P332+P313, P337+P313, P362 | |
| Related compounds | |
Other anions
|
Samarium(III) fluoride Samarium(III) bromide Samarium(III) oxide |
Other cations
|
Samarium(II) chloride Promethium(III) chloride Europium(III) chloride |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Samarium(III) chloride, also known as samarium trichloride, is an inorganic compound of samarium and chloride. It is a pale yellow salt that rapidly absorbs water to form a hexahydrate, SmCl3.6H2O.[1] The compound has few practical applications but is used in laboratories for research on new compounds of samarium.
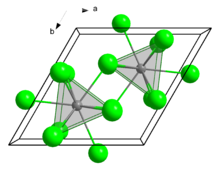
Structure
[edit]Like several related chlorides of the lanthanides and actinides, SmCl3 crystallises in the UCl3 motif. The Sm3+ centres are nine-coordinate, occupying trigonal prismatic sites with additional chloride ligands occupying the three square faces.
Preparation and reactions
[edit]SmCl3 is prepared by the "ammonium chloride" route, which involves the initial synthesis of (NH4)2[SmCl5]. This material can be prepared from the common starting materials at reaction temperatures of 230 °C from samarium oxide:[2]
- 10 NH4Cl + Sm2O3 → 2 (NH4)2[SmCl5] + 6 NH3 + 3 H2O
The pentachloride is then heated to 350-400 °C resulting in evolution of ammonium chloride and leaving a residue of the anhydrous trichloride:
- (NH4)2[SmCl5] → 2 NH4Cl + SmCl3
It can also be prepared from samarium metal and hydrochloric acid.[3][4]
- 2 Sm + 6 HCl → 2 SmCl3 + 3 H2
Aqueous solutions of samarium(III) chloride can be prepared by dissolving metallic samarium or samarium carbonate in hydrochloric acid.
Samarium(III) chloride is a moderately strong Lewis acid, which ranks as "hard" according to the HSAB concept. Aqueous solutions of samarium chloride can be used to prepare samarium trifluoride:
- SmCl3 + 3 KF → SmF3 + 3 KCl
Uses
[edit]Samarium(III) chloride is used for the preparation of samarium metal, which has a variety of uses, notably in magnets. Anhydrous SmCl3 is mixed with sodium chloride or calcium chloride to give a low melting point eutectic mixture. Electrolysis of this molten salt solution gives the free metal.[5]
In laboratory
[edit]Samarium(III) chloride can also be used as a starting point for the preparation of other samarium salts. The anhydrous chloride is used to prepare organometallic compounds of samarium, such as bis(pentamethylcyclopentadienyl)alkylsamarium(III) complexes.[6]
References
[edit]- ^ F. T. Edelmann, P. Poremba (1997). W. A. Herrmann (ed.). Synthetic Methods of Organometallic and Inorganic Chemistry. Vol. 6. Stuttgart: Georg Thieme Verlag.
- ^ Meyer, G. (1989). "The Ammonium Chloride Route to Anhydrous Rare Earth Chlorides—The Example of Ycl 3". The Ammonium Chloride Route to Anhydrous Rare Earth Chlorides-The Example of YCl3. Inorganic Syntheses. Vol. 25. pp. 146–150. doi:10.1002/9780470132562.ch35. ISBN 978-0-470-13256-2.
- ^ L. F. Druding, J. D. Corbett (1961). "Lower Oxidation States of the Lanthanides. Neodymium(II) Chloride and Iodide". J. Am. Chem. Soc. 83 (11): 2462–2467. doi:10.1021/ja01472a010.
- ^ J. D. Corbett (1973). "Reduced Halides of the Rare Earth Elements". Rev. Chim. Minérale. 10: 239.
- ^ Greenwood, Norman N.; Earnshaw, Alan (1984). Chemistry of the Elements. Oxford: Pergamon Press. ISBN 978-0-08-022057-4.
- ^ G. A. Molander, E. D. Dowdy (1999). Shu Kobayashi (ed.). Lanthanides: Chemistry and Use in Organic Synthesis. Berlin: Springer-Verlag. pp. 119–154. ISBN 3-540-64526-8.
