European pied flycatcher
| European pied flycatcher | |
|---|---|

| |
| Adult male in Scotland | |
| Scientific classification | |
| Domain: | Eukaryota |
| Kingdom: | Animalia |
| Phylum: | Chordata |
| Class: | Aves |
| Order: | Passeriformes |
| Family: | Muscicapidae |
| Genus: | Ficedula |
| Species: | F. hypoleuca
|
| Binomial name | |
| Ficedula hypoleuca (Pallas, 1764)
| |
| |
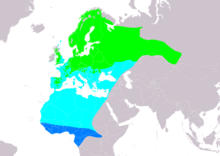
| Range of F. hypoleuca Breeding Passage Non-breeding
| |
| Synonyms | |
The European pied flycatcher (Ficedula hypoleuca) is a small passerine bird in the Old World flycatcher family. One of the four species of Western Palearctic black-and-white flycatchers, it hybridizes to a limited extent with the collared flycatcher.[3] It breeds in most of Europe and across the Western Palearctic. It is migratory, wintering mainly in tropical Africa.[1][4] It usually builds its nests in holes on oak trees.[5] This species practices polygyny, usually bigamy, with the male travelling large distances to acquire a second mate. The male will mate with the secondary female and then return to the primary female in order to help with aspects of child rearing, such as feeding.[3][6]
The European pied flycatcher is mainly insectivorous, although its diet also includes other arthropods. This species commonly feeds on spiders, ants, bees and similar prey.[7]
The European pied flycatcher has a very large range and population size and so it is of least concern according to the International Union for Conservation of Nature (IUCN).[1]
Taxonomy
[edit]The European pied flycatcher is an Old World flycatcher, part of a family of insectivorous songbirds which typically feed by darting after insects.[8] The Latin word ficedula means "small fig-eating bird". The term hypoleuca comes from two Greek roots, hupo, "below", and leukos, "white".[4]
The species was described in Linnaeus's Fauna Svecica (1746), a work that was not binomial and that is therefore unavailable nomenclaturally. Later, in the tenth edition of his Systema Naturae and the next edition of Fauna Svecica (1761), Linnaeus confused this flycatcher with the Eurasian blackcap and the whinchat.[9] To this point, the European pied flycatcher still lacked a proper valid binominal name. The species was finally named as Motacilla hypoleuca by the German naturalist Peter Simon Pallas in 1764. However, he described this species anonymously in the appendix of a sales catalogue of the collection of Adriaan Vroeg, popularly known simply as the "Adumbratiunculae" among ornithologists.[10] The authorship of the Adumbratiunculae would later be attributed to Pallas.[11] Given the initial anonymity of the publication and the inferred authorship by external evidence, the International Code of Zoological Nomenclature advocates that Pallas's name should appear enclosed in square brackets in the species' name. Thus, the correct form of the scientific name of the European pied flycatcher is Ficedula hypoleuca ([Pallas], 1764).[9]
Ficedula hypoleuca currently has three recognized subspecies: the nominate F. h. hypoleuca ([Pallas], 1764), F. h. iberiae (Witherby, 1928), and F. h. tomensis (Johansen, 1916).[9] The subspecies F. h. muscipeta (Bechstein, 1792) is currently considered synonymous with F. h. hypoleuca, but could represent an actual distinct subspecies. The name F. h. atricapilla (Linnaeus, 1766) is a junior subjective synonym of F. h. hypoleuca; and the name F. h. sibirica Khakhlov, 1915 is invalid, the correct form being F. h. tomensis (Johansen, 1916).[9] The very similar Atlas pied flycatcher (Ficedula speculigera), of the mountains of north west Africa was formerly classed as subspecies of the European pied flycatcher.[12][13]
Description
[edit]
This is a 12–13.5 centimetres (4.7–5.3 in) long bird. The breeding male is mainly black above and white below, with a large white wing patch, white tail sides and a small forehead patch. The Iberian subspecies iberiae (known as Iberian pied flycatcher) has a larger forehead patch and a pale rump. Non-breeding males, females and juveniles have the black replaced by a pale brown, and may be very difficult to distinguish from other Ficedula flycatchers, particularly the collared flycatcher, with which this species hybridizes to a limited extent.[14]
The bill is black, and has the broad but pointed shape typical of aerial insectivores. As well as taking insects in flight, this species hunts caterpillars amongst the oak foliage, and will take berries. It is therefore a much earlier spring migrant than the more aerial spotted flycatcher, and its loud rhythmic and melodious song is characteristic of oak woods in spring.
They are birds of deciduous woodlands, parks and gardens, with a preference for oak trees. They build an open nest in a tree hole, and will readily adapt to an open-fronted nest box. 4–10 eggs are laid.[5]
Distribution and habitat
[edit]The European pied flycatcher has a very large range and population size, and is thus deemed to be of least concern by the IUCN. This species occupies areas of many different countries in Europe and northern Africa, also being present in the west Asian portion of Russia. More specifically, the nominate subspecies F. h. hypoleuca inhabits the UK, central Europe and Scandinavia, F. h. iberiae inhabits in the Iberian Peninsula, and F. h. tomensis in eastern Europe and Russia.[9] The species is noted as a vagrant species in places in other countries in Africa and South Asia, such as Sudan and Afghanistan.[1] This flycatcher typically spends winter in tropical Africa.[4][9]
The European pied flycatcher is a terrestrial bird,[1] typically inhabiting open forests, woodlands, and towns. In 2005, the European population was listed to hold 3–7 million pairs.[4]
Mating systems
[edit]The European pied flycatcher predominately practices a mixed mating system of monogamy and polygyny. Their mating system has also been described as successive polygyny.[6] Within the latter system, the males leave their home territory once their primary mates lays their first eggs. Males then create a second territory, presumably in order to attract a secondary female to breed. Even when they succeed at acquiring a second mate, the males typically return to the first female to exclusively provide for her and her offspring.[3] Males will sometimes care for both mates if the nests of the primary and secondary female are close together. The male may also care for both mates once the offspring of the primary female have fledged. The male bird usually does not exceed two mates, practicing bigamy. Only two cases of trigyny have been observed.[15]
Gender difference in mating behavior
[edit]The male mating behavior has two key characteristics: desertion of the primary female and polyterritoriality. The males travel large distances, an average of 200–3,500 metres (660–11,480 ft), to find his second mate. After breeding with the secondary female, the males return to their first mate. The males of this species are polyterritorial; the males will acquire multiple nest sites to attract a female. Upon breeding with this first female, the male will procure more nesting sites, typically some distance from the site of the primary female, in order to attract a second female for mating. The males that have better success at polygyny are typically larger, older and more experienced at arriving earlier to the mating sites.[16]

The female behaviour has also been studied in depth, especially due to the fact that some females accept polygyny while others are able to maintain monogamous relationships. The first female in a polygynous relationship does not suffer much in comparison to females in monogamous situations. These primary females gain greater reproductive success because they are able to secure full-time help from the male once he returns from his search for a second mate. The second female, however, often suffers from polygyny. These females have 60% less offspring than females that are in a monogamous relationship.[17] These findings are consistent with the polygyny threshold model, which is depicted at the right. Additionally, the secondary female lays a smaller clutch which she is more likely to be able to rear on her own.
Another behavior that is relatively frequent in European pied flycatchers is the practice of extra-pair copulations (EPC). Thus, the male practicing EPC will have a group of offspring raised successfully without any parental investment on his part. The female may benefit from EPC if the second male is judged to have superior genes to the original male. Another benefit that EPC adds is that there is an increase in genetic variability. However, females are not typically very welcoming of EPC. A female that is being pursued for an EPC will either passively allow the male to copulate with her, or will resist it and risk injury due to the male's aggression.[18]
Breeding dispersal
[edit]In an experiment conducted from 1948 to 1964 in the Forest of Dean in Gloucestershire, two hundred and fifty nest boxes were carefully recorded for their locations and then analyzed for their inhabitance.[19] The median breeding dispersal (the distance between successive nests) of the European pied flycatcher ranges from about 52–133 metres (171–436 ft), with the average distance between nest sites being about 45 metres (148 ft). This distance typically depended on the breeding density in each year. The study found little evidence to suggest a difference in breeding dispersal between years or between monogamous and polygynous males. As a result, the data for the separate categories could be combined. The breeding dispersal over longer distances could result in both mate fidelity as well as mate change, the latter of which occurs either while the previous mate is still alive, or following the death of the mate. The breeding dispersal distances of birds that survive more than three breeding seasons were studied, and the results showed that the site fidelity increased with more successive breeding attempts. The same long-term study also found that older European pied flycatchers, both male and female, were more likely to move shorter distances between breeding seasons than younger birds were. When mates were observed to re-establish their pair bond, they tended to occupy certain areas that were near the nest site established in the previous breeding season. In addition, female birds were less likely to return to a former breeding site following the death of, or divorce from, their former partner. When a pair divorces, the females have been observed to move greater distances away than the males. As a result, females that keep the same mates from year to year end up moving shorter distances for each mating period than those that divorce. Divorce has little influence on the likelihood of males moving away from their original nest site. The study found that males that keep the same mate do not move significantly smaller distances than males that divorce.[19]
Evolution of polygyny
[edit]Since most bird species exhibit monogamous mating behaviors, the polygynous behavior of the European pied flycatcher has sparked much research. There are three main hypotheses that seek to explain why females settle polygynously when it lowers their overall fitness and reproductive success compared to a monogamous relationship.[20]
"Sexy son" hypothesis
[edit]

The first hypothesis is the "sexy son" hypothesis which asserts that although females experience an initial reproductive loss with their first generation, the reproductive success of the second generation compensates for the initial loss. The second generation of males is thought to be privileged because it will inherit the increased mating ability, or attractiveness, from their fathers and thus will have high success in procuring mates upon maturation. Since these "sexy sons" are projected to have heightened reproductive success, the secondary female's reproductive success in turn improves.[21] Some researchers, however, have refuted this theory, stating that offspring born to secondary females suffered from poor nutrition, which resulted in shorter tarsi and lower weights than the progeny of primary and monogamous females. These phenotypic traits contribute to lesser success in mate acquisition, rejecting the "sexy son" hypothesis.[15]
Deception hypothesis
[edit]The second hypothesis claims that deception from the male flycatcher explains a female's choice to mate with an already-mated male despite the relative decrease in reproductive success.[16] The deception arises from the polyterritoriality of the males, meaning that the males are able to deceive the females through the use of separate territories. This hypothesis attempts to describe why males have developed polyterritorial behavior. The typical long distances between nest sites suggest that males acquire multiple nest sites to facilitate the deception of the secondary female.[15] A study showed that females leave the male upon discovering that he is already mated, as long as she discovers this fact before laying season.[16] However, another experiment with European pied flycatchers in Norway produced results that refute the deception hypothesis.[22] The secondary female birds in their study raised larger clutches than primary females. The study also showed that deception is not an evolutionarily stable strategy for males, because secondary females would notice the frequent visits to the primary females and then elect to choose another mate. According to the deception hypothesis, already-mated males display polyterritorial behavior that increases their chances of acquiring another mate. Unmated males were shown to display mating behavior, consisting mostly of singing, at their nest site. On the other hand, already-mated males would need to disrupt their singing at their secondary territories in order to return to their primary nest. This can occur both before and after the time of their second mating.[23] As a result, it decreases the chance that females would be deceived, leading to an evolutionarily unstable strategy.[24]
Female aggression hypothesis
[edit]
The third hypothesis asserts that females settle for polygyny because it is hard to find unmated males.[22][25] This theory assumes that there is aggression between females to find mates and asserts that polyterritoriality actually helps to alleviate this aggression, allowing the second female a place to settle and breed peacefully.[26] Although the deception hypothesis suggests that males are more successful at farther secondary territories because they can hide their marital status, the female-female aggression suggests that males occupy distant secondary territories to reduce aggression between the primary and secondary females. Primary females display aggression and prevent other females from settling near the initial nest to ensure that they acquire the male parental care.[27] Primary females were seen in experiments to visit the second territory and behave aggressively towards the secondary female. The number of such visits decreased with increasing distance between the nests. It is also important for the primary female to be able to detect an intruding female as soon as possible, because the longer the intruder has been present in a territory, the more difficult it will be to evict the female. Female flycatchers are known to have the capacity to identify the songs of their own mates and check if they establish a second territory. The primary male was also shown to spend less time in the second territories during incubation periods than before they acquired a secondary mate, especially with greater distances between the two territories.[28][29]
Speciation by reinforcement
[edit]F. hypoleuca (pied flycatcher) and F. albicollis (collared flycatcher) are speciating from each other, providing evidence for speciation by reinforcement (selection against hybrid).[30] The two species diverged less than two million years ago, which is considered recent on the time scale of evolution.[31] Still, hybrids of the two species already suffer from low fertility [32][33] and metabolic dysfunction.[34] It was also believed that sexual selection causes reinforcement and pied flycatcher evolved different colouration in sympatry versus allopatry to prevent hybridization, though some evidence suggests heterospecific competition instead of reinforcement as the underlying mechanism.[35] Mating choice tests of the species find that females of both species choose conspecific males in sympatry, but heterospecific males in allopatry [36] (see conspecific song preference). The patterns could suggest mimicry, driven by interspecific competition; [37] however, song divergence has been detected that shows a similar pattern to the mating preferences.[38]
Parental care
[edit]
Studies were also done to examine the amount of contribution the male European pied flycatcher provided in parental care as well as why some females choose to mate with mated males.[39] When older and younger monogamous males were compared, there was no difference in feeding rate between each nest. When females were studied, scientists found that monogamous and primary females benefited significantly more from the male in terms of parental care than polygynous females did. The latter group could only partially compensate for the absence of a male, leading to secondary females and widows raising fewer offspring than the monogamous pairs did. In the study, differences in mates and the qualities of the territories were slight and therefore not considered, since they lead to no advantages for females to choose between the territories belonging to monogamous or already-mated males. The results of the study suggest that the males can control multiple territories and are thus able to deceive females into accepting polygyny, while the females do not have enough time to discover the marital status of the males.
In terms of male parental care to clutches, the rate of male incubation feeding was directly related to the physical condition of the males, and negatively correlated with the ambient temperature. Polygynously mated females also received far less feeds than monogamously mated females, despite having no difference in the food delivery rates by the male. The reduction in delivery rate to the polygynously mated females led to a negative effect on their incubation efficiency, because the females needed to spend more time away from the nest acquiring food. This also prolonged the incubation period when compared to monogamous females. The male feeding behavior is related to the reproductive value as represented by the nests, as well as to the costs and benefits of incubation feeding.[40]
Feeding
[edit]The main diet of the European pied flycatcher is insects. In fact, their name comes from their habit of catching flying insects, but they also catch insects or arthropods from tree trunks, branches, or from the ground.[41] Studies have found that the majority of food catches were made from the ground. It was also found that airborne prey were captured more during the early part of the season (May to June) than in the later part (August to September); the converse trend appeared in prey taken from trees. There are also many overlaps in the foraging techniques with the collared flycatcher, the spotted flycatcher, and the common redstart.[7][42]
Courtship feeding, or incubation feeding, occurs when the male feeds the female in the pairing, egglaying stages, and incubation. An interpretation of this behavior is that it strengthens the pair bond between mates.[43]
Diet
[edit]
The diet of the European pied flycatcher is composed nearly entirely of insects. One study analyzed the stomach contents of birds during the breeding season and found that ants, bees, wasps and beetles made up the main diet.[7] Ants made up approximately 25% of the diet.[44] Food given to nestlings include spiders, butterflies, moths, flies, mosquitoes, ants, bees, wasps, and beetles. For Lepidoptera and Hymenoptera, larvae appear to be consumed more than adult insects; the opposite is true for other insect orders.[42] There is also variation between the proportions of larvae and adult insects between different habitats. Nestlings were also found to consume more spiders, butterfly, and moth larvae, while adult flycatchers consume more ants.[7]
Status
[edit]It has on average decreased in population by 25% within the last 25 years. It has ceased to breed in several parts of its former range within Britain. It is a very rare and irregular breeder in Ireland, with only one or two pairs recorded as breeding in most years.[45] Records of its location can be found on that National Biodiversity Network.[46] In the Netherlands it has declined by 90% due to nestling peaks mistiming.[47]
Lifecycle
[edit]
- mid-September to mid-April: lives in sub Saharan Africa
- mid April to end of May: migrates and arrives in countries such as the United Kingdom
- June to August: breeding season, one brood only
- August to mid September: flies back to sub Saharan Africa
Management and conservation
[edit]They breed in upland broadleaf woodland. This means that in Britain they are limited due to geography to the North and West. They prefer mature oak woodland, but also breed in mature upland ash and birch woods.
They require very high horizontal visibility - a low abundance of shrub and understorey, but with high proportion of moss and grass. Grazing needs to be managed to maintain this open character, but also allow the occasional replacement trees.
They will sometimes use mature open conifer woodland where natural tree holes occur. Generally they prefer trees that have tree holes, i.e. dead trees, or dead limbs on healthy trees. They also like lichens that grow on trees.
Grant funding for conservation
[edit]The Forestry Commission offers grants under a scheme called England's Woodland Improvement Grant (EWIG); as does Natural England's Environmental Stewardship Scheme.
References
[edit]- ^ a b c d e BirdLife International. (2018). "Ficedula hypoleuca". IUCN Red List of Threatened Species. 2018: e.T22709308A131952521. doi:10.2305/IUCN.UK.2018-2.RLTS.T22709308A131952521.en. Retrieved 24 June 2024.
- ^ Gould, John (1837). The Birds of Europe. Vol. 2. (Plate 63 and following text)
- ^ a b c Silverin, Bengt (1980). "Effects of long-acting testosterone treatment on freeliving pied flycatchers, Ficedula hypoleuca, during the breeding period". Animal Behaviour. 28 (3): 906–912. doi:10.1016/s0003-3472(80)80152-7. ISSN 0003-3472. S2CID 53177170.
- ^ a b c d Robinson, R.A. (2005). "Pied Flycatcher Ficedula hypoleuca". BTO BirdFacts. British Trust for Ornithology. Retrieved 4 August 2017.
- ^ a b "RSPB Pied Flycatcher Information Page". RSPB. Retrieved 24 September 2013.
- ^ a b von Haartman, Lars (1951). "Successive Polygamy". Behaviour. 3 (4): 256–274. doi:10.1163/156853951x00296.
- ^ a b c d Silverin, B.; G. Andersson (1984). "Food composition of adult and nestling Pied Flycatchers, Ficedula hypoleuca, during the breeding period (in Swedish with English summary)". Var Fagelvarld. 43 (3): 517–524.
- ^ "Flycatcher". The Columbia Encyclopedia, 6th ed. 2012. Encyclopedia.com. Retrieved December 19, 2012.
- ^ a b c d e f Salvador, Rodrigo B.; Henk van der Jeugd; Barbara M. Tomotani (2017). "Taxonomy of the European Pied Flycatcher Ficedula hypoleuca (Aves: Muscicapidae)". Zootaxa. 4291: 171–182. doi:10.11646/zootaxa.4291.1.10.
- ^ Sherborn, C. Davies (1905). "The new species of birds in Vroeg's catalogue, 1764". Smithsonian Miscellaneous Collections. 47: 332–341 [336 No. 156].
- ^ Rookmaaker, L.C.; Pieters, F.F.J.M. (2000). "Birds in the sales catalogue of Adriaan Vroeg (1764) described by Pallas and Vosmaer". Contributions to Zoology. 69 (4): 271–277. doi:10.1163/18759866-06904005.
- ^ Mayr, Ernst; Cottrell, G. William, eds. (1986). Check-List of Birds of the World. Vol. 11. Cambridge, Massachusetts: Museum of Comparative Zoology. pp. 336–337.
- ^ Gill, Frank; Donsker, David; Rasmussen, Pamela, eds. (December 2023). "Chats, Old World flycatchers". IOC World Bird List Version 14.1. International Ornithologists' Union. Retrieved 24 June 2024.
- ^ Parkin, David T. (2003). "Birding and DNA: species for the new millennium". Bird Study. 50 (3): 223–242. Bibcode:2003BirdS..50..223P. doi:10.1080/00063650309461316.
- ^ a b c Alatalo, Rauno V.; Arne Lundberg (1984). "Polyterritorial polygyny in the pied flycatcher Ficedula hypoleuca - evidence for the deception hypothesis". Annales Zoologici Fennici. 21: 217–228.
- ^ a b c Alatalo, Rauno V.; Carlson, Allan; Lundberg, Arne; Ulfstrand, Staffan (1981). "The Conflict Between Male Polygamy and Female Monogamy: The Case of the Pied Flycatcher Ficedula hypoleuca". The American Naturalist. 117 (5): 738–753. doi:10.1086/283756. S2CID 85184225.
- ^ Lundberg, Arne; Rauno V. Alatalo; Allan Carlson; Staffan Ulfstrand (1981). "Biometry, habitat distribution and breeding success in the pied flycatcher ficedula hypoleuca". Ornis Scandinavica. 12 (1): 68–79. doi:10.2307/3675907. JSTOR 3675907.
- ^ Alatalo, Rauno V.; Karin Gottlander; Arne Lundberg (1987). "Extra-pair copulations and mate-guarding in the polyterritorial pied flycatcher, Ficedula hypoleuca". Behaviour. 1. 101 (3): 139–155. doi:10.1163/156853987X00404.
- ^ a b Harvey, P.H.; Greenwood, P.J.; Campbell, B.; Stenning, M.J. (1984). "Breeding dispersal of the pied flycatcher (Ficedula hypoleuca)". Journal of Animal Ecology. 2 (3): 727–736. Bibcode:1984JAnEc..53..727H. doi:10.2307/4655. JSTOR 4655.
- ^ Orians, G. H. (1969). "On the evolution of mating systems in birds and mammals". The American Naturalist. 12 (3): 589–603. doi:10.1086/282628. S2CID 85112984.
- ^ Weatherhead, Patrick J.; R.J. Robertson (1979). "Offspring quality and the polygyny threshold: the sexy son hypothesis". The American Naturalist. 113 (2): 201–208. doi:10.1086/283379. S2CID 85283084.
- ^ a b Stenmark, Geir; Tore Slagsvold; Jan T. Lifjeld (1988). "Polygyny in the pied flycatcher, Ficedula hypoleuca: a test of the deception hypothesis". Animal Behaviour. 36 (6): 1646–1657. doi:10.1016/s0003-3472(88)80105-2. ISSN 0003-3472. S2CID 53146095.
- ^ Lampe, H.M.; T. Slagsvold (1994). "Individual recognition based on male song in a female bird". Ornithology. 13 (2): 163–164.
- ^ Dale, S; T. Slagsvold (1994). "Polygyny and deception in the pied flycatcher: can females determine male mating status?". Animal Behaviour. 48 (5): 1207–1217. doi:10.1006/anbe.1994.1353. ISSN 0003-3472. S2CID 53162817.
- ^ Dale, Svein; Amundsen, T.; Lifjeld, J.T.; Slagsvold, T. (1990). "Mate Sampling Behaviour of Female Pied Flycatchers: Evidence for Active Mate Choice". Behavioral Ecology and Sociobiology. 27 (2): 87–91. doi:10.1007/BF00168450. S2CID 43744589.
- ^ Davies, Nicholas B., John R. Krebs, and Stuart A. West. An Introduction to Behavioural Ecology. 4th ed. John Wiley & Sons, 2012. Print. ISBN 978-1-4051-1416-5
- ^ Kilpimaa, J.; Alatalo, R.V.; Ratti, O.; Siikamaki, P. (1995). "Do pied flycatcher females guard their monogamous status?". Animal Behaviour. 50 (3): 573–578. doi:10.1016/0003-3472(95)80120-0. S2CID 53160752.
- ^ Slagsvold, T; T. Amundsen; S. Dale; H. Lampe (1992). "Female-female aggression explains polyterritoriality in male pied flycatchers". Animal Behaviour. 32 (3): 397–407. doi:10.1016/s0003-3472(05)80100-9. ISSN 0003-3472. S2CID 53263821.
- ^ Slagsvold, T.; S. Dale (1995). "Polygyny and female aggression in the pied flycatcher: a comment on Ratti et al". Animal Behaviour. 23 (3): 167–175. doi:10.1016/0003-3472(95)80144-8. S2CID 53201140.
- ^ Noor, Mohamed A. F. (1999). "Reinforcement and other consequences of sympatry". Heredity. 83 (5). The Genetics Society (Nature): 503–508. doi:10.1038/sj.hdy.6886320. ISSN 0018-067X. PMID 10620021. (ORCID: 0000-0002-5400-4408 GS: 5nkhrpUAAAAJ).
- ^ Ellegren, Hans; Smeds, Linnéa; Burri, Reto; Olason, Pall I.; Backström, Niclas; Kawakami, Takeshi; Künstner, Axel; Mäkinen, Hannu; Nadachowska-Brzyska, Krystyna; Qvarnström, Anna; Uebbing, Severin (November 2012). "The genomic landscape of species divergence in Ficedula flycatchers". Nature. 491 (7426): 756–760. Bibcode:2012Natur.491..756E. doi:10.1038/nature11584. ISSN 0028-0836. PMID 23103876. S2CID 4414084.
- ^ Ålund, Murielle; Immler, Simone; Rice, Amber M.; Qvarnström, Anna (2013-06-23). "Low fertility of wild hybrid male flycatchers despite recent divergence". Biology Letters. 9 (3): 20130169. doi:10.1098/rsbl.2013.0169. ISSN 1744-9561. PMC 3645050. PMID 23576780.
- ^ Svedin, Nina; Wiley, Chris; Veen, Thor; Gustafsson, Lars; Qvarnström, Anna (2008-03-22). "Natural and sexual selection against hybrid flycatchers". Proceedings of the Royal Society B: Biological Sciences. 275 (1635): 735–744. doi:10.1098/rspb.2007.0967. ISSN 0962-8452. PMC 2596841. PMID 18211878.
- ^ McFarlane, S. Eryn; Sirkiä, Päivi M.; Ålund, Murielle; Qvarnström, Anna (2016-09-01). Proulx, Stephen R (ed.). "Hybrid Dysfunction Expressed as Elevated Metabolic Rate in Male Ficedula Flycatchers". PLOS ONE. 11 (9): e0161547. Bibcode:2016PLoSO..1161547M. doi:10.1371/journal.pone.0161547. ISSN 1932-6203. PMC 5008804. PMID 27583553.
- ^ Vallin, Niclas; Rice, Amber M.; Bailey, Richard I.; Husby, Arild; Qvarnström, Anna (April 2012). "Positive Feedback Between Ecological and Reproductive Character Displacement in a Young Avian Hybrid Zone". Evolution. 66 (4): 1167–1179. doi:10.1111/j.1558-5646.2011.01518.x. PMID 22486696. S2CID 13238049.
- ^ Stre, Glenn-Peter; Moum, Truls; Bureš, Stanislav; Král, Miroslav; Adamjan, Martin; Moreno, Juan (5 June 1997). "A sexually selected character displacement in flycatchers reinforces premating isolation". Nature. 387 (6633): 589–592. Bibcode:1997Natur.387..589S. doi:10.1038/42451. ISSN 0028-0836. S2CID 4363912.
- ^ Coyne, Jerry A. (2004). Speciation. H. Allen Orr. Sunderland, Mass.: Sinauer Associates. p. 361. ISBN 0-87893-091-4. OCLC 55078441.
- ^ Wallin, Lars (2008-04-03). "Divergent character displacement in the song of two allospecies: the Pied Flycatcher Ficedula hypoleuca, and the Collared Flycatcher Ficedula albicollis". Ibis. 128 (2): 251–259. doi:10.1111/j.1474-919X.1986.tb02672.x.
- ^ Alatalo, R.V.; A. Lundberg; K. Stahlbrandt (1982). "Why do pied flycatcher females mate with already-mated males?". Animal Behaviour. 30 (2): 585–593. doi:10.1016/s0003-3472(82)80072-9. ISSN 0003-3472. S2CID 53179058.
- ^ Lifjeld, J.T.; T. Slagsvold (1990). "Manipulations of male parental investment in polygynous pied fly-catchers". Behavioral Ecology. 23 (5): 171–181. doi:10.1093/beheco/1.1.48.
- ^ Lundberg, Arne (2010). The Pied Flycatcher. A&C Black. pp. 55–59. ISBN 978-1408137802.
- ^ a b von Haartman, L. (1954). "Der Traeurfliegenschnaaper". Die Nahrungsbiologic. 83 (1): 1–96.
- ^ Lack, D. (1940). "Courtship feeding in birds". The Auk. 57 (2): 169–178. doi:10.2307/4078744. JSTOR 4078744.
- ^ Bibby, C.J.; R.E. Green (1980). "Foraging behaviour of migrant pied flycatchers, Ficedula hypoleuca, on temporary territories". Animal Ecology. 49 (2): 507–521. Bibcode:1980JAnEc..49..507B. doi:10.2307/4260. JSTOR 4260.
- ^ Newton, S.F. "Rare Breeding Birds in Ireland in 2014 and 2015" Irish Birds Vol.10 p. 234
- ^ "Grid map of records on the Gateway for Pied Flycatcher (Ficedula hypoleuca)". NBN Gateway. Archived from the original on 29 October 2016. Retrieved 24 September 2013.
- ^ Christiaan Both; Sandra Bouwhuis; C. M. Lessells; Marcel E. Visser (2006). "Climate change and population declines in a long-distance migratory bird" (PDF). Nature. 441 (7089): 81–83. Bibcode:2006Natur.441...81B. doi:10.1038/nature04539. PMID 16672969. S2CID 4414217.
External links
[edit]- Ageing and sexing (PDF; 5.7 MB) by Javier Blasco-Zumeta & Gerd-Michael Heinze
- Avibase Archived 2019-09-13 at the Wayback Machine
- Oiseaux Images.
- RSPB Website Description
- Song of a Pied Flycatcher - a British Library sound recording
- Male Pied Flycatcher Song - YouTube video filmed in northern Sweden in May

